Two groundbreaking Israeli ventures – one recruiting dogs and Artificial Intelligence for early detection, and the other engineering T cells to activate the immune system – are harnessing bio-convergence to deliver advanced medical solutions and generating a real transformation in the battle against cancer
In the race to save lives, timing is everything. In a world where most cancer cases are detected too late, the Israeli biotech company SpotItEarly offers a solution that could change reality. This solution comes as a kit with a simple breath test in the form of a face mask, which is capable of detecting the presence of a cancerous tumor before the onset of clinical symptoms.
The alerts indicating the possible presence of cancer are issued by dogs trained to identify cancer-related scent signatures in breath samples. But the innovation doesn’t stop there. SpotItEarly combines the dogs’ exceptional sensory abilities with an advanced AI system that translates their smelling patterns into precise medical data.
The technology integrates biology, machine learning, personalized medicine and, an ambitious vision to save lives by making early cancer screening accessible to extensive populations, including in areas that lack advanced medical infrastructure.
Udi Bobrovsky, a serial entrepreneur with a rich high-tech management background, previously founded and led several companies in the cyber, healthcare and analytics sectors. In 2020, following personal experiences that led him to explore early diagnostics, he became one of SpotItEarly’s four co-founders who all shared a single goal: to save lives.
Supported by the Innovation Authority, the company is currently developing its system in collaboration with medical centers in Israel and the United States, with an initial focus on detecting breast and lung cancers, two of the more fatal types of cancer, which are often diagnosed too late.
“The canine ability to identify cancer-related scent signatures with a high degree of sensitivity has been studied scientifically for 25 years,” explains Bobrovsky. “Dogs have an extremely sophisticated nose and a sense of smell that is far more advanced than any other analytical sensor. No commercial electronic sensor achieves these levels.” SpotItEarly is working to turn that capability into a product that will transform the healthcare industry.
“The dogs arrive at our center at about one year of age and are taught to detect cancer through a unique training process that takes about six months,” says Bobrovsky. The dogs are expected to work a maximum of two to four hours a day, while an entire team ensures their well-being at every level.
“The training process follows protocols we developed and tested. We present the dogs with samples, and they learn to identify the cancerous ones and the type of cancer involved. Furthermore, the technology we are developing, including hardware, software, extensive data collection, and AI, allows us to scale and achieve significantly higher precision. All cancer types share a common scent signature, but there are also distinguishing factors that allow for distinction between different types, such as molecular composition and the scent of specific molecules. In the future, we will be able to provide results for multiple cancer types from a single patient sample.”
Four-legged Sensors
How does it work? After placing an order and receiving a prescription, the breath test kit, which contains a sampling device in the form of a face mask similar to those used during the COVID pandemic, is sent to the user’s home. The user breathes into the mask for about three minutes, then places the sample into a special container that protects it. The sample is then sent to the company’s lab in Israel, where it is analyzed, and the result is delivered within a few days.
“Our sensors, i.e., the dogs, need less than a second to give their indication about a sample,” explains Bobrovsky. “What takes time is the user’s appointment and the timing of the tests, and that’s what our technological system was built to address. It uses a unique algorithm we developed to present the sample at the right moment, automatically, using innovative and groundbreaking equipment developed in our labs.”
Unlike existing cancer screening methods such as colonoscopy or mammography, the breath test, which can detect various types of cancer at a pre-cancerous stage, is painless, non-invasive, nonthreatening, and relatively low-cost. It can be performed easily at home, in a clinic, or at a mobile unit. “In terms of user experience, we want to create something entirely different – one that encourages users to repeat the test regularly, according to each individual’s relevant timeline,” says Bobrovsky.
Moreover, unlike the current system that requires a separate test for each type of cancer, SpotItEarly’s sampling kit will be able to provide results for several different cancer types. A significant advantage is that the kit’s transportation doesn’t require medical staff or refrigeration, making it suitable for distribution in remote or underdeveloped markets.
The company’s vision is simple, says Bobrovsky – to save lives. In that sense, the product’s two main advantages directly serve that purpose: it’s easy to use and able to detect multiple cancer types. “Today, participation in cancer screening is relatively low. We want to bring more people into the cancer screening circle,” he explains. “The more people we reach, the more we can provide them peace of mind with a negative cancer signal, and for those who need it, detection at an early stage and significantly increased chances of beating the disease.”
SpotItEarly has already conducted a large-scale clinical study, which concluded at the end of 2024 and focused on the four most common types of cancer – breast, lung, prostate, and colorectal. The study yielded impressive results, even in the early stages of the disease. “We achieved accuracy levels of nearly 94 percent in Stages 1 and 2,” he says.
“These are the early stages, when the potential for successful treatment is highest. Early detection allows for intervention, significantly increasing the chance of saving the patient and reducing treatment costs. Those requiring further monitoring will be referred to the existing healthcare system, where many will receive appropriate treatment.”


The Goal: Millions of Screening Tests
Alongside its medical and social potential, SpotItEarly’s technology poses significant regulatory challenges. “We have a solution that pushes the boundaries of current regulation,” agrees Bobrovsky, “but we’re in ongoing contact with the Ministry of Health and other regulatory bodies to address those challenges. The Innovation Authority is investing in us through its R&D Fund and Bio-Convergence Program – and are accompanying us throughout the development process, both technologically and from a regulatory perspective.”
Over the past year, the company initiated two further clinical trials, with the objective of completing the regulatory process during the next year and entering the market. The first product, set to be launched next year, will focus on early detection of breast cancer, and will be closely followed by a product for detecting lung cancer. “We want to reach a point where people can order our kit directly, take the test, and receive an answer within just a few days,” says Bobrovsky.
According to Bobrovsky, within five years, the company will operate a solution together with insurance companies, on the scale of millions of samples. SpotItEarly’s goal is to enable routine cancer screening for consumers, either directly or via their insurers, and to refer those with positive signals for further diagnostics to confirm or rule out the results. This approach will allow national healthcare systems investing in extensive resources to reduce costs.
SpotItEarly’s solution is expected to transform everything we know and think about cancer screening: non-invasive and painless tests that are accessible to millions, easy to perform anywhere, inexpensive to distribute, and most importantly, accurate. “It’s a straightforward product, but one that will completely change how we approach cancer screening,” Bobrovsky concludes. “Our kit will bring many more people into the screening cycle, allow them to do it on a regular basis and, if necessary, detect the disease early and save their lives.”
“The life sciences industry in Israel is robust, but the war has raised challenges that require a systemic response. The Innovation Authority acted swiftly, launching investment programs in which digital health, rehabilitation, and medical technologies companies competed for and were awarded investments of approximately half a billion shekels over the past year.”
Dror Bin, CEO, Israel Innovation Authority
Recruiting the Immune System
Another company harnessing bio-convergence to the fight against cancer is Edity Therapeutics, founded in 2020 near the Science Park in Rehovot. Operating at the intersection of therapeutic protein engineering, Artificial Intelligence, and genetic therapies, the company is focusing on one of the most pressing challenges in advanced oncology: the limited efficacy of cell therapies in solid tumors.
While existing CAR-T treatments have achieved dramatic results in blood cancers, they face significant challenges with solid tumors such as pancreatic, ovarian, and colorectal cancers. Edity proposes a novel technological approach that expands the capabilities of engineered T cells, enabling them not only to detect tumors but also to trigger a controlled inflammatory response at the tumor site. This response prompts the body to react as if to an infection, effectively rallying the entire immune system to the attack.
If successful, this technology could transform targeted, limited therapies into a systemic response with the potential to cure cases where conventional medicine has failed.
The concept was developed by Dr. Michal Golan-Mashiach, a computational biologist who emerged from the world of personalized medicine and research. With a background in computer science, business management, and biology, Dr. Golan-Mashiach worked in the fields of high-tech and venture capital before shifting focus to study bioinformatics at the Weizmann Institute.
Her broad experience, from high-tech to bioinformatics and consultation for medical startups, proved instrumental when she was asked to help a cancer patient and develop a personalized disease treatment. “I told myself we need a platform that can take the strongest immune cells and cause them not only to identify tumors, but also to activate a response that mobilizes the entire body,” she explains.
This experience sparked a new insight: If T cells can be engineered not only to deploy their destructive qualities against cancer cells but also to deliver a protein capable of triggering a systemic immune response, the result could have a far more powerful and focused immunological effect.
This innovation was made possible by bio-convergence – a close integration of disciplines once considered separate: biology, engineering, computer science, AI, and materials science. Edity’s solution combines advanced protein engineering, deep understanding of cancer mechanisms, and the engineering capabilities required to introduce therapeutic receptors and proteins into human cells while operating with precise timing. Without recent scientific and technological advances and the integration of science, medicine and technology, such treatment would not have been possible.
Edity’s platform is based on engineering T cells extracted from a patient’s blood and processed in a GMP-certified laboratory. Two key components are introduced into the cells: a receptor capable of focused cancer cells recognition and a therapeutic protein that remains inactive until the cancer cell is identified.
Once the tumor is identified, the protein is activated and initiates a localized inflammation that recruits the immune system en masse to attack the site. Instead of acting on a purely molecular level, the treatment initiates a targeted immune response against the tumor. Dr. Golan-Mashiach emphasizes the uniqueness of the system’s ability to activate the protein with such precision, noting that no other approach currently combines cell engineering and controlled inflammation in this way.
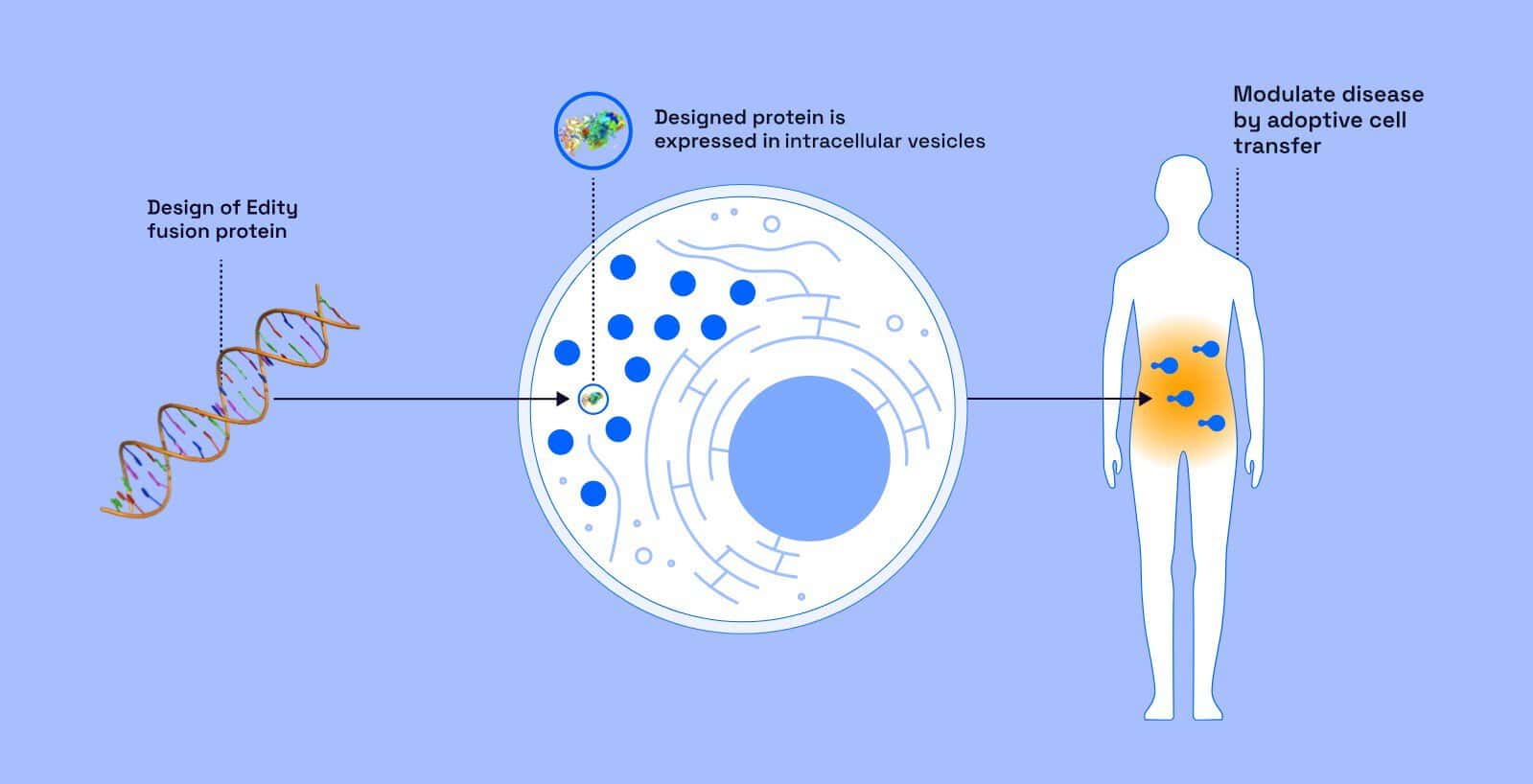
Beyond the scientific aspect, bio-convergence also enables dramatic reductions in development timelines, including algorithms for target discovery, tools for testing cell function in lab conditions, and automation of biological engineering processes that save critical time on the path to achieving clinical outcomes.
Rather than relying solely on trial and error, Edity uses a model in which biological data is analyzed in real time to help shape the treatment’s structure. The result: safer, repeatable, and more precise therapies.
The Aspiration: A New Standard for Public Care
Edity’s development is based in Israel, with preclinical trials currently being conducted in India through a strategic collaboration with Dr. Reddy’s, one of the world’s largest biopharma companies. This collaboration is central to the company’s business model, encompassing not only R&D but also clinical testing, direct investment, and joint operations.
Today, Edity’s work is spread across three main locations: the development center in Rehovot, with laboratories adjacent to the Weizmann Institute; the operational site in India, where preclinical trials are currently conducted before planned human trials; and a small management team in Boston, led by Dr. Golan-Mashiach and the company’s president.
The vast majority of the team – scientists, researchers, and operations personnel – is based in Israel, where most of the engineering, development, and testing processes are carried out. “The entire process up to the stage of animal testing happens here,” she emphasizes. “It’s not just sentiment but rather, a deep belief in our ability to engage in top-tier science outside Silicon Valley.”
Two initial clinical trials are scheduled to begin in 2027 in both Israel and India – one targeting ovarian cancer and the other colorectal cancer. In both cases, the patients selected will have exhausted existing treatment options, including chemotherapy and biological immunotherapy.
According to the company, approximately half of all ovarian cancer patients are expected to qualify for the new treatment, based on the tumor’s biological profile. The clinical protocol includes blood sampling and a biopsy from the patient, engineering of T cells, quality control tests, and reinfusion of the cells under inpatient care, with close monitoring to prevent severe immune responses.
While such responses, also known in CAR-T therapies, are seen on the one hand as proof of biological efficacy, they also require strict medical monitoring. The company believes that its conditionally active protein may help focus the immune response, while reducing the associated risks.
“Our goal isn’t just to prove that the technology works, but to do so in a way that enables treatment at an affordable cost,” says Dr. Golan-Mashiach. “Today’s genetic therapies can cost half a million dollars or more. Most people in the world can’t afford that, and it’s simply not sustainable for healthcare systems.”
Edity’s solution could be a game-changer in global healthcare, moving from laboratory promises to real-world capabilities for treating tumors previously considered untreatable. If the company meets its cost and efficacy goals, it could transform cancer care not only clinically, but also on a systemic-social level.
To achieve this goal, the company committed early on to simplifying production, shortening logistics, and building a protocol that would also be suitable for public healthcare systems. “We’re doing it in just two weeks,” says Dr. Golan-Mashiach, “not because we cut corners scientifically, but because we understand that the system must evolve. If it works, there’s no reason this can’t become the new standard.”
Redefining the Balance Between Power and Risk
Edity’s vision is to develop a groundbreaking therapy and also to build a manufacturing process that, from the beginning, supports large-scale implementation. This vision led the company to join the Israel Innovation Authority’s Bio-Convergence program, as part of the global collaboration with India.
The Authority has supported Edity since its earliest stages – from the initial ‘Tnufa’ Program to more advanced tracks – even when the core technological concept was still in early development. “In our first applications, we presented the idea as a general platform,” recalls Dr. Golan-Mashiach. “Only later did we realize the immense potential, specifically in cancer, and that’s where we decided to start. The Innovation Authority helped us refine, focus and advance.”
The collaboration with Dr. Reddy’s was made possible, in part, thanks to the regulatory context enabled by the program: connecting a clinical need in a developing country to Israeli innovation, that is designed from the outset to be more affordable and faster to implement.
In many ways, the treatment Edity is striving to bring to the market represents a milestone in what is now called “biologically personalized medicine”: not just genetic matching, but a timed immune response directed at turning the body into an active partner.
This isn’t necessarily the shortest or cheapest therapy, but if it works, it could shift the balance between power and risk. “When I see the difference in response between standard T cells and the engineered ones in our lab mice, I’m filled with renewed purpose,” says Dr. Golan-Mashiach. “It’s much more than research, it’s like witnessing the first signs of a life-saving possibility.”
What does success look like for Dr. Golan-Mashiach? First, receiving approval for human trials. Next, clinical success. And ultimately, making the treatment accessible, and its inclusion in national healthcare coverage, and availability to patients awaiting a real solution.
“I’m not looking to sell the company the day after a breakthrough,” she emphasizes. “My goal is to build a long-term, stable company here in Israel – one that delivers the next generation of cutting-edge medical solutions. I want to employ the country’s top researchers and maintain the company’s value locally. If one day Edity becomes the biotech equivalent of what Teva was for generics – we’ll know we’ve succeeded.”
“Bio-convergence is not just a technological scientific development. By integrating biology, engineering, and Artificial Intelligence, we are creating technologies that can save lives and transform entire systems.”
Dror Bin, CEO, Israel Innovation Authority
